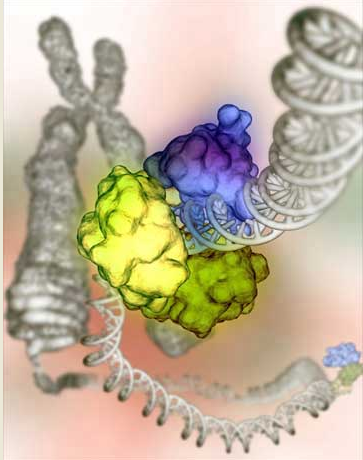
Base Excision Repair (BER)
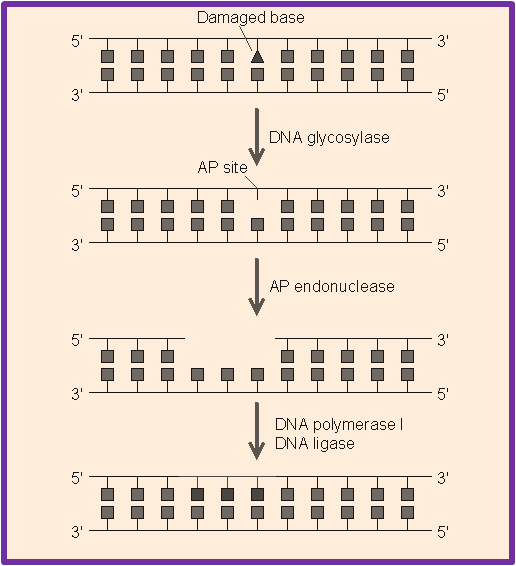 BER plays a mojor role in removing small, non-helix-distorting base lesions from the genome that could otherwise cause mutations by mispairing or lead to breaks in DNA during replication. BER is initiated by DNA glycosylases, which recognizes and removes specific damaged or inappropriate bases, forming AP sites. These are then cleaved by an AP endonuclease . The resulting single-strand break can then be processed by the process of BER.
BER plays a mojor role in removing small, non-helix-distorting base lesions from the genome that could otherwise cause mutations by mispairing or lead to breaks in DNA during replication. BER is initiated by DNA glycosylases, which recognizes and removes specific damaged or inappropriate bases, forming AP sites. These are then cleaved by an AP endonuclease . The resulting single-strand break can then be processed by the process of BER.
The various base lesions repaired by BER includes:
- Oxidized bases: 8-oxoguanine, 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyG, FapyA)
- Alkylated bases: 3-methyladenine, 7-methylguanine
- Deaminated bases: hypoxanthine formed from deamination of adenine. Xanthine formed from deamination of guanine
- Uracil inappropriately incorporated in DNA or formed by deamination of cytosine
In addition to base lesions, the downstream steps of BER are also utilized to repair single-strand breaks.
There are a number of proteins that are involved in BER pathway including MBD4, NEIL1, MUTYH, SMUG1, etc. The Deletions in some BER genes increases the mutation rate in a variety of organisms, predicting that loss of BER could contribute to the development of cancer. Mutations in the DNA glycosylase MYH are also known to increase susceptibility to Colon cancer .
Nucleotide Excision Repair (NER)
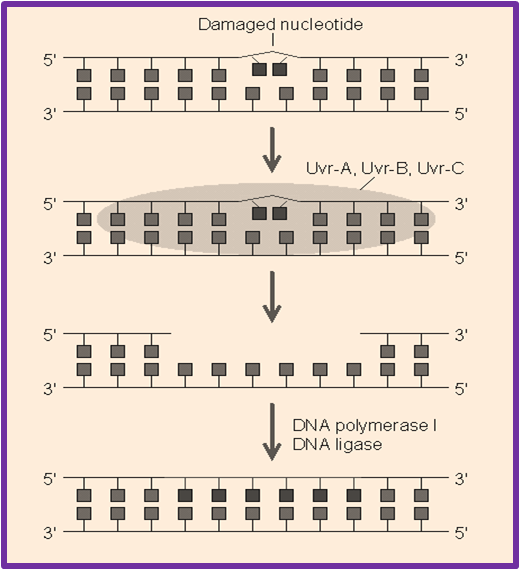 NER is an important mechanism by which the cell can prevent unwanted mutations by removing the vast majority of UV-induced DNA damage (mainly thymine dimers and 6-4-photoproducts). This mechanism mostly repairs bulky helix-distorting lesions as these NER enzymes recognize bulky distortions in the shape of the DNA double helix. Recognition of these distortions leads to the removal of a short single-stranded DNA segment that includes the lesion, creating a single-strand gap in the DNA, which is subsequently filled in by DNA polymerase, which uses the undamaged strand as a template for synthesis. NER is an important mechanism by which the cell can prevent unwanted mutations by removing the vast majority of UV-induced DNA damage (mainly thymine dimers and 6-4-photoproducts). This mechanism mostly repairs bulky helix-distorting lesions as these NER enzymes recognize bulky distortions in the shape of the DNA double helix. Recognition of these distortions leads to the removal of a short single-stranded DNA segment that includes the lesion, creating a single-strand gap in the DNA, which is subsequently filled in by DNA polymerase, which uses the undamaged strand as a template for synthesis.
There are 9 major proteins involved in NER in mammalian cells and their names come from the diseases associated with the deficiencies in those proteins. XPA, XPB, XPC, XPD, XPE, XPF, and XPG all derive from Xeroderma pigmentosum and CSA and CSB represent proteins linked to Cockayne syndrome . Additionally, the proteins ERCC1, RPA, RAD23A, RAD23B, and others also participate in nucleotide excision repair. The importance of this repair mechanism is evidenced by the severe human diseases that result from in-born genetic mutations of NER proteins including Xeroderma pigmentosum and Cockayne's syndrome.
Mismatch Repair (MMR)
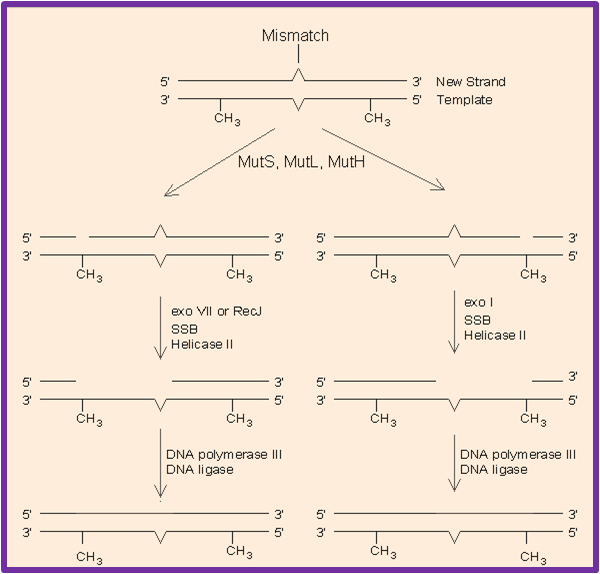 MMR recognizes and repairs erroneous insertion, deletion and mis-incorporation of bases that can arise during DNA replication and recombination, as well as repairs some forms of DNA damage.This process of repair is strand-specific and the mismatched bases including G/T or A/C pairing and other mismatches are commonly due to tautomerization of bases during synthesis.The damage is repaired by recognition of the deformity caused by the mismatch, determining the template and non-template strand, and excising the wrongly incorporated base and replacing it with the correct nucleotide.
MMR recognizes and repairs erroneous insertion, deletion and mis-incorporation of bases that can arise during DNA replication and recombination, as well as repairs some forms of DNA damage.This process of repair is strand-specific and the mismatched bases including G/T or A/C pairing and other mismatches are commonly due to tautomerization of bases during synthesis.The damage is repaired by recognition of the deformity caused by the mismatch, determining the template and non-template strand, and excising the wrongly incorporated base and replacing it with the correct nucleotide.
There are a number of important mismatch repair proteins but three of these proteins are essential in detecting the mismatch and directing repair machinery to it- MutS, MutH and MutL. Mutations in the human homologues of the Mut proteins affect genomic stability, which can result in Microsatellite instability (MI) . MI is implicated in most Human cancers specifically Hereditary nonpolyposis colorectal cancers (HNPCC) which is attributed to mutations in the genes encoding the MutS and MutL homologues MSH2 and MLH1. A subtype of HNPCC is also known as Muir-Torre Syndrome (MTS) which is associated with Skin tumors.
Homologous Recombination Repair (HRR)
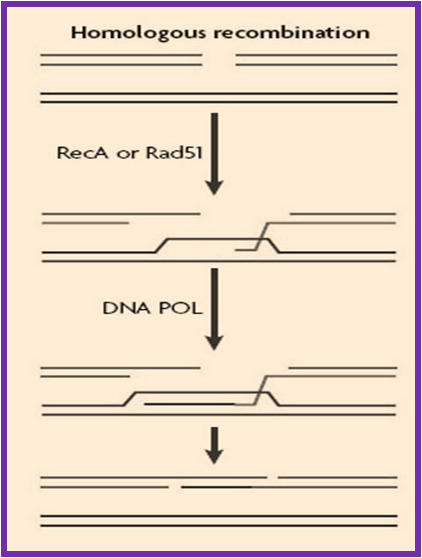 Homologous recombination is mainly a genetic recombination in which nucleotide sequences are exchanged between two similar or identical molecules of DNA. This mechanism is mostly used by the cells to accurately repair harmful breaks that occur on both strands of DNA, known as double-strand breaks that are caused by ionizing radiation or DNA-damaging chemicals. If these breaks remain unrepaired, this can cause large-scale rearrangement of chromosomes in somatic cells which may in turn lead to Cancer.
Homologous recombination is mainly a genetic recombination in which nucleotide sequences are exchanged between two similar or identical molecules of DNA. This mechanism is mostly used by the cells to accurately repair harmful breaks that occur on both strands of DNA, known as double-strand breaks that are caused by ionizing radiation or DNA-damaging chemicals. If these breaks remain unrepaired, this can cause large-scale rearrangement of chromosomes in somatic cells which may in turn lead to Cancer.
Thus, recombination provides critical support for DNA replication in the recovery of stalled or broken replication forks, contributing to tolerance of DNA damage. There are many HRR proteins like Rad51, BRCA1 which play important role in the repair mechanisms and abberations in these genes have been implicated in a number of disorders including Genomic instability and contributes to cancer etiology. Mutations in the BRCA2 recombination gene causes predisposition to Breast and Ovarian cancer as well as Fanconi anemia , a cancer predisposition syndrome characterized by a defect in the repair of DNA interstrand crosslinks.
Non-Homologous End Joining (NHEJ)
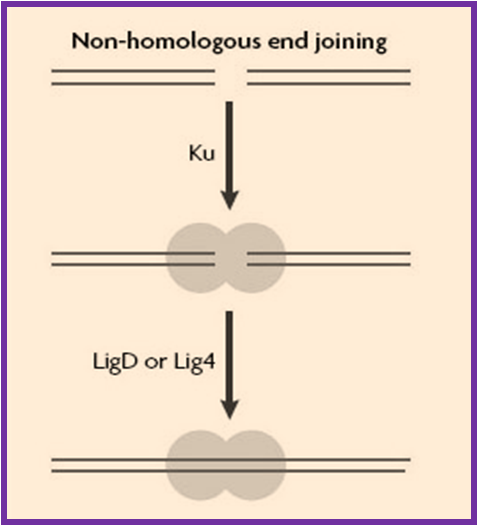 Non-homologous end joining (NHEJ) pathway mainly repairs the double-strand breaks (DSB's) in DNA, which if not repaired or misrepaired, can result in mutations, chromosome rearrangements and eventually in cell death. This pathway is named as "non-homologous" since the broken ends are ligated directly without the presence of a homologous template whereas in HRR, a homologous sequence is required to guide the repair. In mammals DSB's are primarily repaired by NHEJ and HRR, while HRR
is mainly found in yeast and NHEJ can repair almost all kind of cells.
Non-homologous end joining (NHEJ) pathway mainly repairs the double-strand breaks (DSB's) in DNA, which if not repaired or misrepaired, can result in mutations, chromosome rearrangements and eventually in cell death. This pathway is named as "non-homologous" since the broken ends are ligated directly without the presence of a homologous template whereas in HRR, a homologous sequence is required to guide the repair. In mammals DSB's are primarily repaired by NHEJ and HRR, while HRR
is mainly found in yeast and NHEJ can repair almost all kind of cells.
Deficiencies in DSB repair are associated with hereditary diseases such as Nijmegen breakage syndrome , Ataxia telangiectasia , Bloom’s syndrome and Breast cancer , etc. Several human syndromes are associated with the malfunctioning of NHEJ pathway. Mutations in LIG4 and XLF cause LIG4 syndrome and XLF-SCID. As per the experimental evidences, deletion of XRCC4 or LIG4 in mice causes embryonic lethality which confirms that NHEJ pathway is indispensible for the viability in mammals.
|